INTERMEDIATE PART -II
CHEMISTRY PAPER - II
Time Allowed: 20: Minutes
Maximum Marks: 17
OBJECTIVE
NOTE: You have four choices for each objective type a question as A, B, C and D. The choice which you think is correct, fill that circle in front of that question number. Use marker or pen to fill the circles. Cutting or filling two more circle will result in zero mark in that question. Attempt as many question as given in objective type question paper and leave other blank. Write the letter A, B, C and D in the column (write correct option) against each question. If there is a contradiction in the bubble and hand written answer, bubble option will be considered correct.Four possible answer to each statement are given below. Choose the correct answer and encircle it.
Question#1
- Preparation of vegetable ghee involves:
(a) Halogenation
(b) Hydroxylation
(c) Hydrogenation
(d) Dehydrogenation
- Benzene cannot undergo:
(a) Substitution reaction
(b) Elimination reaction
(c) Addition reaction
(d) Oxidation reaction
- When CO2 is made to react with Ethyl Magnesium Iodide, followed by acid Hydrolysis, the product formed is
(a) Propane
(b) Propanoic acid
(c) Propanal
(d) Proparal
- Which enzyme is not invohed in formation of Starch?
(a) Diastase
(b) Zymase
(c) Urease
(d) Invertase
- Which of the following will have the highest hailing point?
(a) Methanol
(b) Ethanal
(c) Propanal
(d) Hexaone
- Which or the Following compounds will react with Tollen’s reagent?
(a) 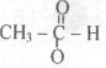
(b) 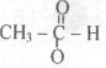
(c) 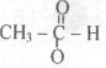
(d)
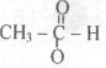
- A carboxylic Acid contain:
(a) A hydroxyl group
(b) A carboxyl group
(c) A hydroxyl and carboxyl group
(d) A carboxyl and Aldehydic group
- Which of the following is Addition polymer?
(a) Polystyrene
(b) Nylon 6, 6
(c) Terylene
(d) Epoxy resin
- Which is not a Calcareous Material?
(a) Lime
(b) Marble
(c) Clay
(d) Marine shell
- The pH of acid rain is:
(a) 7- 6.5
(b) 6 -5
(c) 6- 6.5
(d) Less than 5
- Which statement is incorrect?
(a) All the metals are good conductor of electricity
(b) All the metals are good conductor of heat
(c) All the metals form positive Ions
(d) All the metals form Acidic Oxides
- Chile Saltpetre has the chemical formula:
(a) NaNO3
(b) KNO2
(c) Na2B4O7
(d) Na2CO3H2O
- The brown gas formed when metal reduces HNO3 is:
(a) N2O5
(b) NO2
(c) NO
(d) N2O
- Tincal is mineral of:
(a) Al
(b) Si
(c) B
(d) C
- Bleaching powder may he produced by passing chlorine over:
(a) Calcium carbonate
(b) Hydrated calcium sulphate
(c) Anhydrous calcium sulphate
(d) Calcium hydroxide
- Which of the following is typical transition metal?
(a) Co
(b) Se
(c) Y
(d) Ra
- Linear shape is associated with which set of Hybrid orbital
(a) Sp
(b) Sp2
(c) Sp3
(d) dsp
INTERMEDIATE CART -II
CHEMISTRY PAPER –II
Time Allowed: 2:40 Minutes
Maximum Marks: 68
Subjective
NOTE: Write short answers of any twenty-two questions of Section - 1 and attempt any three questions from Section – 11.Write same questions number and its part number in answer book, as given in the Question Paper.
Section-1
2. Attempt any eight parts 8 x 2 =16
- State Modern Periodic Law.
- Define Hydration Energy, Give an example.
- The reaction of an Alkali medal oxide with walet is an acid base reaction and not an oxidation reduction reaction.
- Complete the following reactions:-
(i) Mg(NO3)2 →
(ii) BeO + NaOH →
- Why aqueous solution of Na2CO3 is Alkaline in nature?
- What is Teflon? Give its two uses.
- Give four uses of Borax.
- What are Silicones? Write Formula of Methyl Silicone.
- What arc Semiconductors?
- What are Chelates? Give an example_
- Write down structural Formulae of Pyrrole and Anthracene.
- What are Functional groups? Name functional groups of Alcohols and Ethers
3. Attempt any eight parts. 8 x 2 =16
- Give two reactions For the preparation or Phosphorus Acid (H3PO3)
- Give four points of similarity of Oxegen and Sulphur.
- How will you prepare n-butane from an Alkyl halide?
- What is Raney Nickel? How is it prepared?
- What are Polycyclic Aromatic Hydrocarbons? Give two examples.
- Give a reaction showing Aceylation of Benezene.
- What are Primary and Secondary Aklkyl Halides Give one example of each.
- Why dry ether is necessary for the preparation of Gringnard Reagent?
- Give formula of Paraldehyde and Metaformaldehyde.
- Give four uses of Acetaldehyde.
- What is Rancidity of fats and oils and what is its cause?
- How many types of Nucleic acids are present and what is their fuuction?
3. Attempt any six parts. 6×2 =12
- What products are obtained by the Oxidation of 2-propanol and 2-methyl-2- propanol.
- What is the order of reactivity of primary, secondary and tertiary alcohols when C- O bond and O — H bond break?
- Why Phenol is acidic in nature?
- Give reactions when Phenol reacts with
(a) Conc. HNO3 and (b) Conc. H2SO4
- Give the mechanism for the formation of Acetamide from Acetic acid and Ammonia.
- Give two reactions of a-amino acids.
- What are fertilizers and why are they needed?
- What is the average composition of Lime, Silica, Alumina and Magnesium Oxide in a good Portland Cement.
- How water is made potable by Coagulation?
SECTION-II
Note: Attemp any three questions.
Question#5
(a) Define Halides. Give their classification. 4
(b) Explain peculiar of Beryllium. 4
Question#6
(a) Explain the term Reforming of petroleum. 4
(b) Using Ethyl bromide as a starting material, how would you prepare the following Compounds.
(i) n-Butane
(ii) Ethyl alcohol
(iii) Ethyl Cyanide
(iv) Ethene
Question#7
(a) Write a note on Acidic Nature of Acetylene? Prove it by two reactions. 4
(b) Describe mechanism of reaction of Acetone with sodium Bisulphite. 4
Question#8
(a) What are Friedel and Craft's reactions? Give it two examples along with Mechanism 4
(b) How would you convert Acetic Acid into the following compounds?4
(i) Acetyl Chloride
(ii) Acetamide
(iii) Ethyl acetat
(iv) Ethane
Question#9
(a)Define Sacrificial Corrosion and explain Electrochemical Theory of Corrosion. 4
(b) Describe the process of Incineration and Industrial Waste. 4