(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
RAWALPINDI BOARD 2012
CHEMISTRY PART-I
Time: 20 MM.
(Objective Part)
Marks: 17
Note: You have four choice for each objective type question as A, B, C and D. The choice which you think is correct; fill that circle in front of that question number. Use marker or pen to fill the circles. Cutting or filling two or more circles will result in zero mark in that question.
(a) 8 g of Oxygen
(b) 16 g of Oxygen
(c) 32 g of Oxygen
(d) 24 g of Oxygen
(a) 3.6g of H2O
(a) 5.4g of N2O5
(c) 4.8g of C2H5OH
(d) 2.8g of CO
(a) Cooled very slowly to get large size crystals.
(b) Cooled at fast rate to get medium sized crystals.
(c) Evaporated to get crystals of the product
(d) Mixed with immiscible liquid to get pure crystals of the product.
(a)

(b)
(c)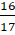
(d)
(a)
X 1023
(b) 55.6 X 1023
(c)X 1023
(d)X 1023
(a) enhanced electronegative character of nitrogen.
(b) Pyramidal structure of NH3
(c) Ion pair of electrons present on nitrogen.
(d) very small size of nitrogen atom.
(a) covalent crystal
(b) Ionic crystals
(c) Amorphous cyrstals
(d) Molecular crystals
(a) 7d
(b) 7f
(c) 7q
(d) 7s
(a) Stark effect
(b) Compton effect
(c) Zeeman effect
(d) Photo electric effect
(a) F2
(b) N2-2
(c) O2+2
(d) Br2
(a) Three sigma bond
(b) one sigma and 2π
(c) one sigma and one π
(d) two σ and one π
(a) 4.184 J
(b) 418.4 J
(c) 0.4184 J
(d) 41.84 J
(a) 1.5
(b) 2.0
(c) 3.0
(d) 2.7
(a) 5.1
(b)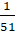
(c)
(d) 3.0
(a) Increases slowly
(b) Decreases slowly
(c) Does not change
(d) Drop to zero
(a) no reaction
(b) Fe is deposited
(c) Cu is oxidized
(d) Fe is reduced
(a) 2
(b) 1
(c) 3
(d) Zero
Time: 2:40 Hours
(Subjective Part)
Marks: 68
SECTION-I
2. Write short answer any Eight Parts.
3. Write short answer any Eight Parts.
4. Write short answer any Six Parts.
SECTION-II
5. (a) Define Stoichiometry. Give its assumptions, Mention any two important laws which help to perform stoichiometric calculations.
(b) What are molecular solids? What types of forces are present in them? Write down their properties.
6. (a) Define Co-ordinate covalent bond. Explain with the help of two examples.
(b) Neutralization of 100cm3 of 0.5M NaOH at 25°C with 100cm3 of 0.5M HCl at 25°C raised the temperature-of reaction mixture to 28.5°C. Fins the enthalpy of Naturalization specific heal of water = 4.2JK-1 g-1.
7. (a) How Neutrons were discovered?
(b) 250cm3 of sample of hydrogen effuses four times as rapidly as 250cm3 of an unknown gas. Calculate molar mass of unknown gas.
8. (a) What is an Ionic product of water? How does this value vary with the change in temperature?
(b) What are electrolytic cells? Explain with diagram and give an example of electrolysis of fused salt
9. (a) Give the graphical explanation for the elevation of boiling point of a solution.
(b) Explain half life method for the measurement of the order of a reaction.