Inter (Part-II) Fedral Board 2014
Chemistry
Part I (Objective Type)
Time Allowed: 20 Minutes
Max. Marks: 17
Note: Four possible answers A, B, C and D to each question are given. The choice which you think is correct, fill that circle in front of that question with Marker or Pen ink in the answer-book. Cutting or filling two or more circles will result in zero mark in that question.
Question#1. Circle the correct option i.e. A/B/C/D. Each part carries one mark.
- Radius of fluorine atom is_________
A. 67 pm
B. 79 pm
C.72 pm
D. 89 pm
- ________is amphoteric Oxide in nature.
A. BeO
B. MgO
C. Li2O
D. CaO
A. V A
B. VI A
C. III A
D. IV A
- Pure Sulphuric acid freezes at:
A. 10.9°C
B. 10.5°C
C. 9.5°C
D. 12.1°C
- Radon is the a -decay product of the ___________.
A. Rubidium
B. Radium
C. Polonium
D. Helium
A. Fled light
B Green light
C. Blue light
D Yellow light
- Bleaching powder when reacts with NH3 produces_____________ gas.
A. O2
B. Cl2
C. H2
D. N2
- Linear shape is associated with which set of hybrid orbitals?
A. sp
B sp2
C. sp3
D. dsp2
- The acid .which is obtained when benzene is oxidized in the presence of V2O5 is:
A. Oxalic acid
B. Formic acid
C. Pitric acid
D. Maleic acid
- CO2 when reacts with C2H5 MgBr gives
A. Butanoic add,
B. Ethanoic acid
C. Propanoic acid
D. Pentanoic acid
- While changing molasses into glucose the enzyme needed is
A. Zymase
B. Diastase
C. Maltase
D. Invertase .
- Paraldehyde is the polymer of:
A. ECHO
B. CH3CHO
C. CH3—CH2--CHO
D. CH3—CH2—CH2—CHO
- Ninhydrin test is used to identify_______________.
A. Fatty acids.
B. Carbohydrates
C. Amino acids
D. Acetic acid
- Benzene-Dicarboxylic acid
A. 1, 2— Benzenedicarboxylic acid
B. 1, 4 Benzenedicarboxylic acid
C. 1, 3— Benzenedicarboxylic acid
D. Propanedioic acid
- Starch is a mixture of two polysacchrides________________:
A. Amylose and Sucrose
B. Amylose and Cellulose
C. Amylose and Glycogen
D. Amylose and Amylopectin
- Which three elements are needed for the healthy growth of plants?
A. N, S, P
B. N, Ca, P
C. N. P, K
D. N, K, C
- The 99.5% mass of the lithosphere is made of ___________elements.
A. 9
B. 10
C. 11
D. 12
CHEMISTRY HSSC-II
SECTION B
Q: 2: Answer any FOURTEEN parts. The answer to each part should not exceed 5 to 6 lines.
- a. Why does H2O Kaye higher boiling point than HF?
b.
Why are the oxides of alkali metals and alkaline earth metals basic in nature?
- a. What are Polymeric halides?
b.
Why does carbon have high melting point?
c.
Define the term "Electron affinity'.
- a. Give the names and formulae of the minerals of Beryllium.
b.
Give the reaction of Be with NaOH. Also give the name of the compound formed.
c.
Why are the ionic hydrides reducing in nature?
- a. How is the lime mortar prepared?
b.
How does gypsum change into Plaster of Paris?
- a. How is borax prepared from Colemanite? Only give reaction with the balanced equation.
b.
How is Boric acid prepared from borax? Give reaction.
c.
Why does Aluminium not react even with conc. HNO3?
- a. What are the allotropes of Phosphorous? Give structure.
b.
Why are the elements of group IV A called chalogens?
c.
What is the role of Testing? Box in the contact process for the preparation of H2SO4?
- a. How can C12O7 be prepared? Give reaction.
b.
Define Disproportion reaction.
c.
Why are the Oxyacids of chlorine stronger than oxyacids of Bromine and iodine?
- a. Give the reaction of bleaching powder with dil H2SO4, and NH3 (give balanced equation of reaction)
b.
Why does the solubility-of the noble gases increase in water with increase in atomic number?
- a. Draw the geometric shape of PCI5. Also give the type of hybridization in it.
b.
What is the use of Aluminium in Bessemer's process?
c.
Give the reaction 'of chromyl chloride test.
- a. Define the Reforming process.
b.
What are alicyclic compounds? Give examples.
- How will you convert:
- Succinic acid ---→Ethene
- Ethene---→ Formaldehyde
- Complete the following reactions with mechanism:
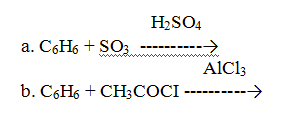
- a. Why is Ethane a gas but Ethanol a liquid?
b.
Why does the solubility of higher alcohol decrease in water?
c.
Why does phenol show acidic nature?
- a. What is Tollen's test? Also give the reaction.
b.
Convert aceton ----------→ acetone hydrazone.
- Predict the product of the following reactions:
(image)
- Explain condensation polymerization with example.
- a. What is Pulp washing process?
b.
What are Micronutrients?
- a. How are Leather Tanneries causing pollution in water?
b.
What is the role of dissolved oxygen in measuring the quality of water?
- a. Briefly explain the reactivity of carbonyl group.
b.
How can acetaldehyde be prepared by the dry distillation of a mixture having calcium salt of formic and acetic acid?
SECTION - C
Note: Attempt any TWO questions. All questions carry equal marks.
Question#3
a. Explain Borax-bead test with chemical reaction.
b. Give peculiar behaviors of Boron.
c. Explain the Electrochemical theory for protecting the metal from corrosion.
Question#4
a. What are β-elimination reactions? How does E1 reaction differ from SN1 reaction?
b.
Give the reactions of C2H5 — Mg — Br with complete mechanism.
- Ethylene Epoxide
- CO2
- Acetone
c. How will you convert the following:
- Phenol Picric acid
- Ethyne acetic acid
- Methylnitrile ------→ acetic acid
Question#5
a. Explain the following:
- Condensation polymerization
- Polyamide Resin
b. Explain bleaching process in the paper industry.
c What is Smog? Give the conditions necessary for smog formation.