Chemistry past paper 2015
Gujranwala Board
Note:you have four choices for each objective type question as A,B,C and D. the choice which you think is correct, fill that circle in front of that question number. Use marker or pen to fill the circles. Cutting or filling two or more coircles will result in zero mark in that question. Attempt as many question as given in objective type question paper and leave others blank.
- Solvent extraction is an equilibrium process and it is controlled by:
(a)Law of mass action
(b) distribution law
(c) the amount of solvent used
(d) the amount of solute
- The mass of one mole of electrons is:
(a)008 mg
(b) 0.55 mg
(c) 0.184 mg
(d) 1.673 mg
- The volume occupied by 1.4g of N2 at S.T.P is:
(a) 24dm3
(b) 22.4 dm3
(c) 1.12dm3
(d) 112dm3
- In zero order reaction, the rate is independent of:
(a) temperature of reaction
(b) concentration of reactants
(c) concentration of products
(d) none of these
- If the salt bridge is not used between two half cells, the voltage:
(a) decreases rapidly
(b) decreases slowly
(c) does not change
(d) drops to zero
- Molarity of pure water is:
(a) 1
(b) 18
(c) 55.5
(d) 6
- pH of pure water is:
(a) 4.4
(b) 5.4
(c) 7.0
(d) 8.0
- The pH of 10-3 mol dm-3 of an aqueous solution of H2SO4 is:
(a) 0
(b) 2.7
(c) 2.0
(d) 1.5
- Calorie is equivalent to:
(a) 0.4184J
(b) 141.84J
(c) 4.184J
(d) 418.4J
- Bond angle
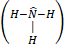 in case of ammonia molecule is:
in case of ammonia molecule is:
(a) 105°
(b) 107.5°
(c) 120°
(d) 180°
- which of the following molecules has zero dipole moment?
(a) NH3
(b) CHCl3
(c) H2O
(d) BF3
- Cathode rays strike alumina and produce a _________ colour.
(a) red
(b) blue
(c) yellow
(d) green
- When 6d orbital is complete, the entering electron goes into:
(a) 7f
(b) 7s
(c) 7p
(d) 7d
- Which of the following is a pseudo solid?
(a) CaF2
(b) Glass
(c) NaCl
(d) All
- London dispersion forces are the only forces present among the:
(a) Molecules of water in liquid state
(b) Atoms of helium in gaseous state at high temperature
(c) Molecules of solid iodine
(d) Molecules of hydrogen chloride gas
- A real ga obeying van der waal’s equation will resemble ideal gas if:
(a) both ‘a’ and ‘b’ are large
(b) both ‘a’ and ‘b’ are small
(c) a is small and b is large
(d) a is large and b is small
- The molar volume of CO2 is maximum at:
(a) S.T.P
(b) 127°C and 1 atm
(c) 0°C and 2 atm
(d) 273°C and 2 atm
Note: Section I is compulsory. Attempt any three(3) questions from sections. II and any three (3) part from section III
(Section-I)
2. Write short answers to any EIGHT questions:
- Differentiate between ion and molecular ion.
- Calculate the mass in grams of 10-3 moles of water.
- Why N2 and CO have the same number of protons, electrons and neutrons?
- How mixture of NH4Cl and NaCl can be separated?
- Define distribution law.
- Calculate the value of gas constant ‘R’ in SI-units.
- Write two faulty assumptions of kinetic molecular theory of gases.
- Describe that burning of candle is a spontaneous process.
- Define system and surrounding with suitable example.
- Define “Le-Chatelier’s principle”.
- Prove that pKa + pKb = 14 at 25°C.
- Define pH and pOH.
3. Write Short answers to any EIGHT question:
- Evaporation causes cooling. Explain.
- Vacuum distillation can be used to avoid decomposition of a sensitive liquid. Explain with reason.
- Diamond is hard and an electrical insulator, why?
- Transition temperature is exhibited by both elements and compounds. Explain.
- Why of cathode rays is equal to that of electrons?
- Cathode rays are material particles. Explain with reason.
- Define Hund’s rule. Give an example.
- Distribute the electron in orbitals of 29Cu and 26
- Why cationic radius radius is smaller than parent atom?
- How does ionization energy vary in a group of periodic table?
- Why CO is polar and CO2 is non-polar?
- π-bonds are more diffused than σ-bonds. Give reason.
4. Write Short answers to any SIX question:
- Why the sum of mole fractions of all components of a solution is always equal to unitly ?
- Why the aqueous solution of NH4Cl is acidic?
- Define ebullioscopic constant with an example.
- Find out the oxidation state of Mn in (a) KmnO4 (ii) K2MnO4
- How impure Cu can be purified by electrolytic process?
- What is the function of salt bridge in galvanic cell?
- How a catalyst is specific in its action?
- Give two characteristics of an enzyme catalysis.
- What is poisoning of a catalyst? Give an example.
(Section-II)
Question 5:
(a)Define vapour pressure and give a method for its measurement (4)
(b)Mg metal reacts with HCl to give hydrogen gas. What is the minimum volume of HCl solution (27% by weight required to produce 12.1g H2? The density of HCl solution is 1.14g cm-3 (4)
Mg(s)+2HCl(aq)→MgCl2(aq)+H2(g)
At. mass of Mg = 24 gmol-1
At. mass of Cl = 35.5 gmol-1
Question 6:
(a)What is an ideal gas? Real gases deviate more from ideal behavior at low temperature and under high pressure. Explain. (4)
(b)How are positive rays produced in discharge tube? Give properties of these rays. (4)
Question 7:
(a)Write down the main postulates of VSEPR theory and discuss the structure of NH3 with reference of this theory. (4)
(b)Define and explain Hess’s law of constant heat summation with examples. (4)
Question 8:
(a)How does the Arrhenius equation help us to calculate the energy of activation of a reaction?
(b)Calculate the pH of a buffer solution in which 0.11 molar CH3COONa and 0.09 molar acetic acid solution are present. Ka value forCH3COOH is 1.85 x10-5 .At mass of C = 12gm/mol O 16 gm/mol H =1 gm/mol Na = 23gm/mol
Question 9:
(a)Write a note on fuel cell.
(b)Explain raoult’s law when both components in the solution (solute and solvent) are volatie.
(Section-III Practical)
Note: (i) Write down standard solution, chemical equation with mole ratio, indicator with end point, procedure and supposed reading with calculation for part A,B &C (1+1+1+1+1)=5
(ii) Write down material required, diagram and procedure for part D & E. (1+1+3)=5
Question 10:
- The given solution contains 6g of a mixture of HCl and NaCl dissolve per dm3. Determine the % age composition of the mixture.(molar mass of HCl = 36.5)
- You are given a solution of KMnO4 which contains 5g KMnO4 per dm3.find out % age purity of the sample. (Molar mass of KMnO4 =158)
- 15 grams of Na2S2O3.5H2O are dissolved per 500 cm3 of solution. Determine the % age purity of the sample. (molar mass of Na2S2O3.5H2O =248)
- How would you crystallize benzoic acid from water?
- Separate mixture of various inks by paper chromatography.
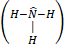 in case of ammonia molecule is:
in case of ammonia molecule is: