CHEMISTRY HSSC-II (2016)
SECTION-A
(Marks 17)
NOTE.- Section-A is compulsory. All parts of this section are to be answered on the question paper itself. It should be completed in the first 25 minutes and handed over to the Centre Superintendent. Deleting/overwriting is not allowed. Do not use lead pencil.Circle the correct option i.e. A/ B/C/ D. Each part carries one mark.
Question#1
- In the organic compounds the carbon atom generally forms:
(A) Hydrogen bond
(B) Ionic bond
(C) Metalic Bond
(D) Covalent bond
- Which of the following oxides is basic in nature:
(A) Al2O3
(B) P4O+0
(C) SO3
(D) Na2O
- Due to inert pair effect, the element of group IV having electronic configuration ns2, np2 will form:
(A) M4+ cation
(B) M+ cation
(C) M2+ cation
(D) M3+ cation
- The oxidation states -1,+1,+3,4-5and +7 are shown by all the halogens except:
(A) Bromine
(B) Iodine
(C) Chlorine
(D) Fluorine
- Pale-green is a characteristic flame colour of:
(A) Sodium
(B) Calcium
(C) Barium
(D) Strontium
- Group VIII elements are generally called:
(A) Halogens
(B) Alkali metals
(C) Noble gases
(D) Coinage elements
- The functional group having ej structure
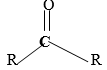
represent the family called
(A) Ketones
(B) Ethers
(C) Esters
(D) Carboxylic acid
- The IUPAC name of the compound HC - C- CH= CH- CH,?
(A) Pnta-3-ene-5-yne
(B) Penta-3-ene-i1-yne
(C) Penta-4-ene-yne
(D) Penta 2 ene-4-yne
- The compounds, n-Butane and isobutene are best considered as:
(A) Chain isomers
(B) Positional isomers
(C) Metamers
(D) Functional group isomers
- Reduction of Alkyl Nitrites gives:
(A) Alcohols
(B) Alkanes
(C) Primary amines
(D) Sec amines
- Acetones can be obtained by the oxidation of:
(A) Propane
(B) Ethanol
(C) 1-propanol
(D) 2-propanol
- The nitration of phenol at 25 produces:
(A) Toluene
(B) O-nitrophenol
(C) Benzene
(D) Phenol nitrate
- The long chains of monosaccharides are called:
(A) Vitamins
(B) Oils
(C) Carbohydrates
(D) Proteins
- Which of the following is NOT an alternative to ozone depleting chlorofluorocarbon (CFCs)?
(A) CO2
(B) Hydrofluorocarbons(HFCs)
(C) Perflourocarbons(PFCs)
(D) Hydrocarbons
- Which of the following technique DOES NOT involve electromagnetic radiation?
(A) Ultraviolet
(B) Infrared
(C) Mass spectroscopy
(D) Nuclear magnetic resonance spectroscopy
- Double bond is formed as a result of:
(A) Polymerization reaction
(B) Substitution reaction
(C) Elimination reaction
(D) Addition reaction
- 3Ca+N2→ :
(A) CaN2
(B) Ca 3N2
(C) Ca3N
(D) Ca2N3
CHEMISTRY HSSC-II (2016)
SECTION-B
(Marks 42)
2. Answer any FOURTEEN parts. The answer to each part should not exceed 5 to 6 lines. (14x3=42)
- (a) Why are the elements of group I called alkali metals?
(b)How do the elements of group I resemble with group II elements? 02
- Write down the chemical reactions of the following elements of 3rd period with chlorine 03
(i) Sodium
(ii) Aluminum
(iii) Silicon
- Briefly discuss the metallic and Non-metallic character of group IV elements 03
- Why is Zinc group included in transit elements? Give reason 03
- What is the trend of following properties of croup VII elements? 03
(i) Atomic radius
(ii) Melting and boiling points
- Write down the procedure for the detection of carbon and hydrogen in the organic compound 03
- (a) Define the term homologous series? 01
(b) Give four characteristics of Homologous series. 02
- How can alkenes be used to prepare? 03
(i) Vicinal dibromide
(ii) alkyl-atocies
(iii) Alkane
- Predict the major product of bromination of following compounds by their reactions 03
(i) Toluene
(ii) Nitrobenzene
(iii) Be.lzene
- Write down condensation reactions
(a) Between two identical ketones 03
(b) Between aldehyde and ketone
- Ctorting from Ethyl chloride. How will you prep-are 03
(i) Ethanol
(ii) Primary Amines
(iii) n-Butane
- How is phenol prepared from? 03
(i) Chiorobenzene
(ii) Sodium Benzene sulphonate.
(iii) Arydiazenium
- (a) What are alkanoic acids? 01
(b) Write down the reaction for the preparation of its two derivations. 02 (xiv) Give step wise mechanism for Alcohol condensation either. 03
- (a) What is the difference between organic and inorganic compounds? 01
(b) Write down four uses of organic compounds in our daily life. 02
- Define and give one example of each the Following 03
(i) Dyes
(ii) Tnermosetting polymers
(iii) Petro Chernicals
- What are Ethers? Give their classification. 01+02
- (a) What is acid rain? 01
(b) Write down two adverse effect of acid rain or our environment 02
- (a) What are proteins? 01
(b) Give two important furctiora of proteins in the human body 02
SECTION-C
(Marks 26)
Note: Attempt any TWO questions carry equal macks. (2 x13= 26)
Question#3
(a) Explan the periodicity of following properties of 3rd period elements of periodic table:
(i) Atomic radius 02
(ii) Ionization Energy 02
(iii) Electrical Conductivity 02
(b) Discuss the trends in solubility of Hydroxides of group II elements 04
(c) What is spectroscopy? Name four spectroscopic techniques used in modern Methods of analysis 01+02
Question#4
(a) Give a flow-sheet diagram for the classification of Hydrocarbons on the basis of structure Also give one example of each type 05
(b) write down the steps of free radical chain mechanism for the bromination of methane. 04
(c) Write down two chemical reactions in which Benzene behaves as an unsaturated Compound 04
Question#5
(a) Distenguish Primary. Secondary and Ternary alcohols with the help of reactions 04
(b) Write down the structures of following compounds 04
(i) Trans-Butene dioic acid
(ii) n-Butyl bromide
(iii) 3-Methyl - 1 - Butyne
(iv) Cycle- 1. 3-hexadrene
Question#6
Explain the following with the hero of suitable examples:
(i) Metamerism 02
(ii) Geometrical Isomerism 03