(b) Write I.U.P.A.C. names of followings: Compounds are given
(i) (CH3)3 – C– OH
(ii) CH3 - O – CH2- CH2 – CH3
(iii)
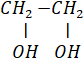
(iv) (CH3)2- CH -
BAHAWALPUR BOARD 2017
PAPER CHEMISTRY PART-2
Time: 20 MM.
(Objective Part)
Marks: 17
Note: You have four choice for each objective type question as A, B, C and D. The choice which you think is correct; fill that circle in front of that question number. Use marker or pen to fill the circles. Cutting or filling two or more circles will result in zero mark in that question.
(A) Metallic character increases down the group
(B) Metallic character increases from left to right along a period
(C) Metallic character remains the some from left to right along a period
(D) Metallic character remains the same down the group
(A) KNO3
(B) NaNO3
(C)Na2B4O7.10H2O
(D) Na2CO3.H2O
(A) Al
(B) B
(C) Si
(D) C
(A) O2
(B) O2+
(C) P2-
(D) Q22-
(A) CIO3
(B) CIO2
(C) Cl2O7
(D) C12O5
(A) 2
(B) 1
(C) 6
(D) 4
(A) sp
(B) sp²
(C) Sp³
(D) dsp²
(A) Methane
(B) Ethene
(C) Ethyne
(D) Propane
(A) HSO41-
(B) SO31+
(C) SO3
(D) H2SO4
(A) Propane
(B) Propanol
(C) Propionic Acid
(D) Acetic Acid
(A) Acid
(B) Acid as well as a Base
(C)Salt
(D) Base
(A) Acetaldehyde
(B) Formaldehyde
(C) Benzaldehyde
(D) Trimethylacetaklehyde
(A) H2/Pt
(B) H2/Ni
(C) LiA1H4
(D) NaBH4
(A) CH2 = CH – CH3
(B) CH3 - CHO
(C) CH3COOC2H5
(D) CH3—CH2-COOH
(A) Both are Insoluble in Water
(B) Both are naturally occurring
(C) Both are Hexoses
(D) Both are Disaccharide
(A) Lime
(B) Marble
(C) Clay
(D) Marine Shell
(A) Chromium (III)
(B) Chromium (V1)
(C) Lead
(D) Copper
BAHAWALPUR BOARD 2017
PAPER CHEMISTRY PART-2
Time: 3:10 Hours
(Subjective Part)
Marks: 83
Section-I
2. Attempt any EIGHT short questions.
3. Attempt any EIGHT short questions.
4. Attempt any SIX short questions.
SECTION-II
Attempt any THREE questions.
5. (a) Discuss variation of Electrical Conductance along Periods and Groups in Periodic Table.
(b) Discuss the Peculiar Behavior of Lithium.
6. (a) Give Systematic Names to following complexes:
(i) [ Pt (OH)2(NH3)4 ]SO4
(ii) [ Cr(.OH)3(H2O)3 ]
(iii) K2 [Cu (CN)4]
(iv) [Fe ( H2O )6 ]
(b) Discuss any two components of Environment.
7. (a) Write a note on sp² Hybridization of Carbon explaining structure of Ethene.
(b) What products are formed when following compounds are treated with Ethyl Magnesium Bromide, followed by Acid Hydrolysis
(i) HCHO
(ii) CH3CHO
(iii) (CH3)2CO
(iv) CO2
8. (a) Prepare Ethane from Kolbe's Electrolytic Method. Also write down its Mechanism.
(b) Write laboratory and Industrial preparation for Acetaldehyde.
9. (a) Give the methods of preparation of Benzene on large scale (any four).
(b) Write I.U.P.A.C. names of followings: Compounds are given
(i) (CH3)3 – C– OH
(ii) CH3 - O – CH2- CH2 – CH3
(iii) 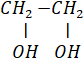
(iv) (CH3)2- CH -![]() - OH
- OH